FLUORESCENT NANODIAMOND (FND) FLOW CYTOMETRY
REQUIRED MATERIAL AND EQUIPMENT
- Fixative solution (4% Paraformaldehyde in PBS)
- Saponin-based permeabilization and wash reagent (10x solution)
- Adherent cells
- Reaction tubes (size depends on the volume of reaction cocktail needed)
- Appropriate cell culture medium
- 1% BSA (bovine serum albumin) in PBS, pH 7.1 – 7.4
- Deionized water or 18 MΩ purified water
- Flow cytometry tubes
WORKFLOW
The following protocol was developed using a fluorescent nanodiamond (FND) volume of 10 μL and can be adapted for any cell type. There are many factors which can influence the labeling such as the growth medium, the density, and the type of cells. To determine the optimal FND concentration for your experiment, a range of concentrations should be tested for your cell type and experimental conditions.
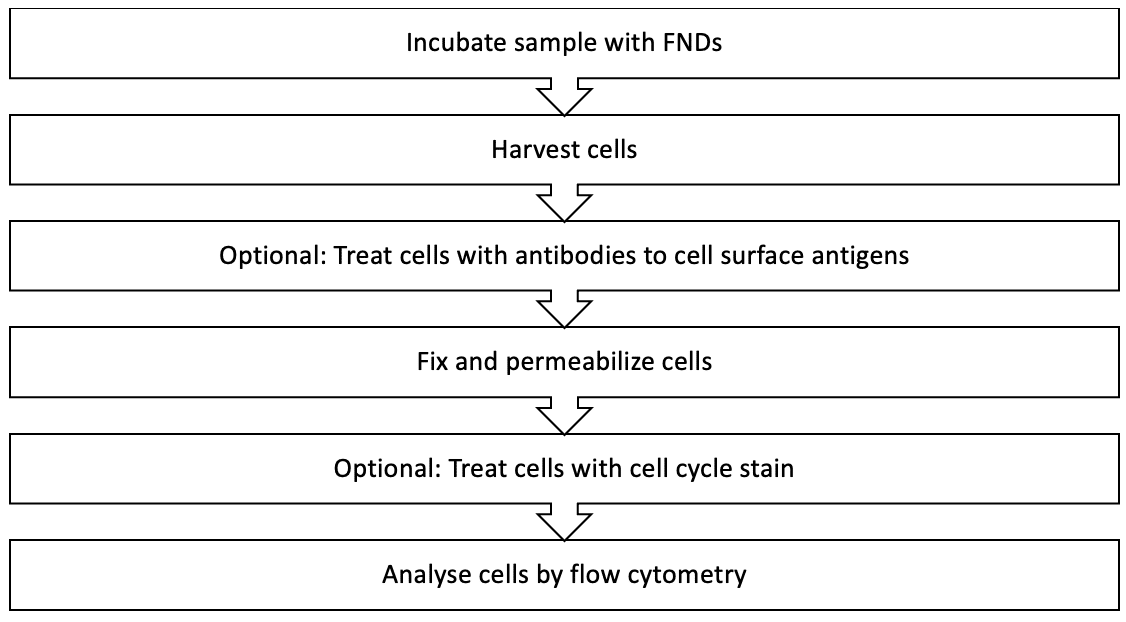
KEY FLOW CYTOMETRY PROTOCOL STEPS
The four main steps in flow cytometry protocols are:
- Sample Preparation: Single-cell suspensions should be prepped through the use of mechanical dissociation methods and detachment techniques such as using enzymatic solutions or calcium chelation reagents.
- Blocking: Anti-Fc antibody dilution is usually applied to suspended cells to prevent nonspecific binding of primary antibody.
- Antibody Incubation: Flow cytometry incubation can involve various components such as primary antibodies, secondary antibodies, streptavidin, and antibody-FND immunoconjugates.
- Data Acquisition: Most flow cytometers are accompanied by the necessary software to obtain and transform the signals from cell characteristics. The user then sets the specific software parameters for their experiment.
SAMPLE PREPARATION
A cell population that can be made into a single cell suspension can be assessed by flow cytometry. The following three points are important to consider:
- To prepare an ex vivo cell population, freshly dissected tissue is often gently homogenized using mechanical dissociation methods, and different cell types are separated via density gradient centrifugation to remove intracellular matrix material, debris, and irrelevant cell populations.
- It is important to note that adherent cells must be detached from cell culture vessel surfaces using enzymatic solutions or calcium chelation reagents prior to use.
- Suspended cell cultures only need to be counted and assessed for viability.
Cell counts/titers refer to viable cells in suspension that can be determined by using an automated cell counter and applying gating to exclude dead cells/debris. Alternatively, viability can be assessed by microscope-aided counting of the number of live cells in a known volume, such as by using a hemocytometer and Trypan blue dye, which is excluded by the intact membrane of live cells, and only the non-viable dead cells uptake this dye.
BLOCKING
Fc receptors can bind antibodies through their Fc region and are present on many cell types. Thus they can can generate increased background or even false positive signals in flow cytometry analysis. To prevent binding of FND-immunoconjugates by Fc receptors, the suspended cells should be pre-treated with a blocking reagent such as specific anti-Fc receptor antibodies, excess purified IgG, or excess (unpurified) IgG in the form of adult serum. This will minimize binding of the Fc or constant region of the FND-immunoconjugates. The immunoconjugate is added immediately at the end of the blocking incubation without a wash step. This ensures that blocking of non-specific antibody binding is maintained throughout.
ANTIBODY INCUBATION
Primary antibody incubation
Unlike other antibody-based applications, such as immunohistochemistry, dilution of antibody for flow cytometry is typically based not on mass of antibody per volume of buffer, but on mass of antibody per number of cells in the sample. As with other applications, an optimal concentration must be empirically determined. Antibody may be diluted in flow cytometry assay buffer. Staining in small volumes improves access of antibody to cells in suspension. At the end of the incubation period, cells should be washed in staining or assay buffer at least three times to remove any unbound primary antibody.
Secondary antibody incubation
If an indirect detection technique is employed, primary incubation is followed by incubation with an appropriate dilution of secondary antibody specific for the isotype of each primary antibody used. In multicolor detection experiments, fluorescent nanodiamonds of sufficiently different wavelengths or colors must be selected for each secondary antibody to permit the differentiation of signal from each target. While antibodies coupled to light-sensitive fluorophores must be protected by incubation in the dark, incubation with fluorescent nanodiamond-coupled secondary antibody can be carried out in the in the light. Samples should be maintained on ice and centrifuged at 4°C.
FLUORESCENT NANODIAMONDS
Many antibodies used in flow cytometry can be directly conjugated to Cymaris fluorescent nanodiamonds; however, many unlabeled primary antibodies are routinely used in combination with labeled secondary antibodies.
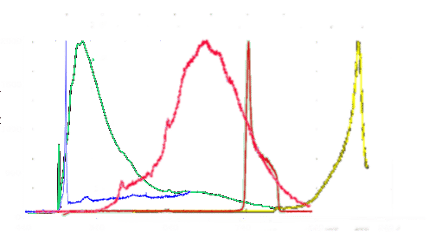
Figure 1. Spectral excitation and emission wavelengths of NV and NVN fluorescent nanodiamonds.
Fixation and storage
Once surface antigen staining has been completed, cells may be fixed in paraformaldehyde in phosphate-buffered saline instead of resuspending for acquisition. This is useful when samples cannot be acquired immediately after staining, as it allows cells to be stored overnight at 4°C. Afterward, the fixative should be diluted, and the cells washed at least twice. Although acquisition immediately following staining is recommended, fixed cells that have been stored at 4°C may be acquired up to 48 hours after fixation.
Cell Permeabilization
When the protein(s) of interest is intracellular, fixation of cells after surface staining is necessary to increase structural strength so that the cells can withstand the subsequent permeabilization needed to allow antibodies access to intracellular antigen. Cells that have been fixed and washed may be permeabilized by incubation with a suitable detergent in phosphate-buffered saline for no more than 15 minutes at room temperature before diluting the detergent solution and washing once. Non-ionic detergents such as saponin are recommended. Permeabilization must be maintained during all steps involving antibodies or streptavidin, and this is achieved by including detergent in the staining buffer throughout subsequent staining up to and including the fluorophore incubation step. Detection of intracellular antigen by flow cytometry otherwise follows the principles and procedures outlined above and may be direct, indirect, or direct/indirect with signal enhancement.
DATA ACQUISITION
Most current flow cytometers are accompanied by the software necessary to acquire and transform the signals generated by the particle characteristics as each cell passes by the detector. These software programs usually include components that aid in organizing the experiment, a feature that is of particular importance when analyzing a variety of samples from multiple specimens.
For multicolor experiments, it is critical to set parameters for compensation, in recognition of the wavelength overlap amongst spectra of NV and NVN nanodiamonds used in the experiment. Compensation calibrates each nanodiamond’s spectrum and allows for subtraction of signals from adjacent channels due to spectral overlap.
